Amnesia collection download. Atomic Number of Copper is 29.
Chemical symbol for Copper is Cu. Number of protons in Copper is 29. Atomic weight of Copper is 63.546 u or g/mol. Melting point of Copper is 1083,5 °C and its the boiling point is 2595 °C.
Symbol: Cu Atomic Number: 29 Atomic Mass: 63.5 Number of Protons/Electrons: 29 Number of Neutrons: 35 Classification:Transition Metals Discovery: A long time ago Discoverer: IDK Uses: electrical conductor. Needing personal assistance from the credit union? Contact the CU from the website or call (740) 289-5060. What is the routing number for Atomic CU? Get the routing number, assets, loans, and other financial information.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearElement Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.546. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. A copper ion with a charge of +2 has 29 protons and 27 electrons. The atomic number of an element is equal to the number of protons in the nucleus of each atom of that element. Symbol: Cu Atomic Number: 29 Atomic Mass: 63.546 amu Melting Point: 1083.0 °C (1356.15 K, 1981.4 °F) Boiling Point: 2567.0 °C (2840.15 K, 4652.6 °F) Number of Protons/Electrons: 29 Number of Neutrons: 35 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 8.96 g/cm 3 Color: red/orange Atomic Structure.
About Copper
Copper is a soft metal resembling gold in its color and texture. It is known as one of the oldest discovered metals, and humanity worked with copper millions of years ago. It has got its current name from a Latin word meaning metal from Cyprus. Copper is essential for living creatures, and we have it in our bodies in small doses, for proper function of our enzymes. It is possible to find copper in its pure form in nature, but it is usually obtained from natural minerals. Centuries ago, copper was considered to be a precious metal and was used to produce coins, along with gold and silver. Besides, copper is extensively used in producing electrical element, especially wires or others. Together with tin, copper forms an alloy named bronze, which is used for producing kitchenware, valves, gears, electrical components, bells, and so on. Another famous alloy of copper – the one with zinc – is called brass, and it is widely used for producing parts of musical instruments, screws, buttons, door knobs, and many other things.
Uses of Copper
Copper, a reddish and ductile metallic element with the symbol Cu, is mainly used as a conductor of electricity and heat. Copper and its useful alloys are employed in the medical field and in agriculture. Cuprous oxide is used as a pigment in glasses, porcelain, paints, and ceramics. Cupric sulfate is mostly used for agricultural purposes. Cuprous chloride is generally used as a catalyst. Cuprous sulfide is used in electrodes, solar cells, etc. Sterling silver, an alloy of silver and copper, is used in forks, spoons, knives, medical instruments, as well as in the manufacture of saxophones. Monel, an alloy of nickel, copper and small amounts of iron, carbon, manganese, silicon, is preferred in aircraft construction, buildings, oil production, piping systems, musical instruments, the chemical industry. Monel is also used in the frames of eyeglasses and even in motion picture film processing.
Compounds with Copper
- Cu2O: Cuprous oxide
- CuO: Cupric oxide
- Cu2Cl2: Cuprous chloride
- Cu2S: Cuprous sulfide
- CuSO4: Cupric sulfate
- CuS: Cupric sulfide
- CuCl2: Cupric chloride
Properties of Copper Element
| Atomic Number (Z) | 29 |
|---|---|
| Atomic Symbol | Cu |
| Group | 11 |
| Period | 4 |
| Atomic Weight | 63.546 u |
| Density | 8.96 g/cm3 |
| Melting Point (K) | 1357.77 K |
| Melting Point (℃) | 1083,5 °C |
| Boiling Point (K) | 2835 K |
| Boiling Point (℃) | 2595 °C |
| Heat Capacity | 0.385 J/g · K |
| Abundance | 60 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 1.9 |
| Ionization Energy (eV) | 7.72638 |
| Atomic Radius | 135pm |
| Covalent Radius | 138pm |
| Van der Waals Radius | 140 |
| Valence Electrons | 1 |
| Year of Discovery | prehistoric |
| Discoverer | unknown |

What is the Boiling Point of Copper?
Copper boiling point is 2595 °C. Boiling point of Copper in Kelvin is 2835 K.
What is the Melting Point of Copper?
Copper melting point is 1083,5 °C. Melting point of Copper in Kelvin is 1357.77 K.

How Abundant is Copper?
Abundant value of Copper is 60 mg/kg.
What is the State of Copper at Standard Temperature and Pressure (STP)?
State of Copper is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure. War for the overworld - heart of gold expansion download.

When was Copper Discovered?
Copper was discovered in prehistoric.
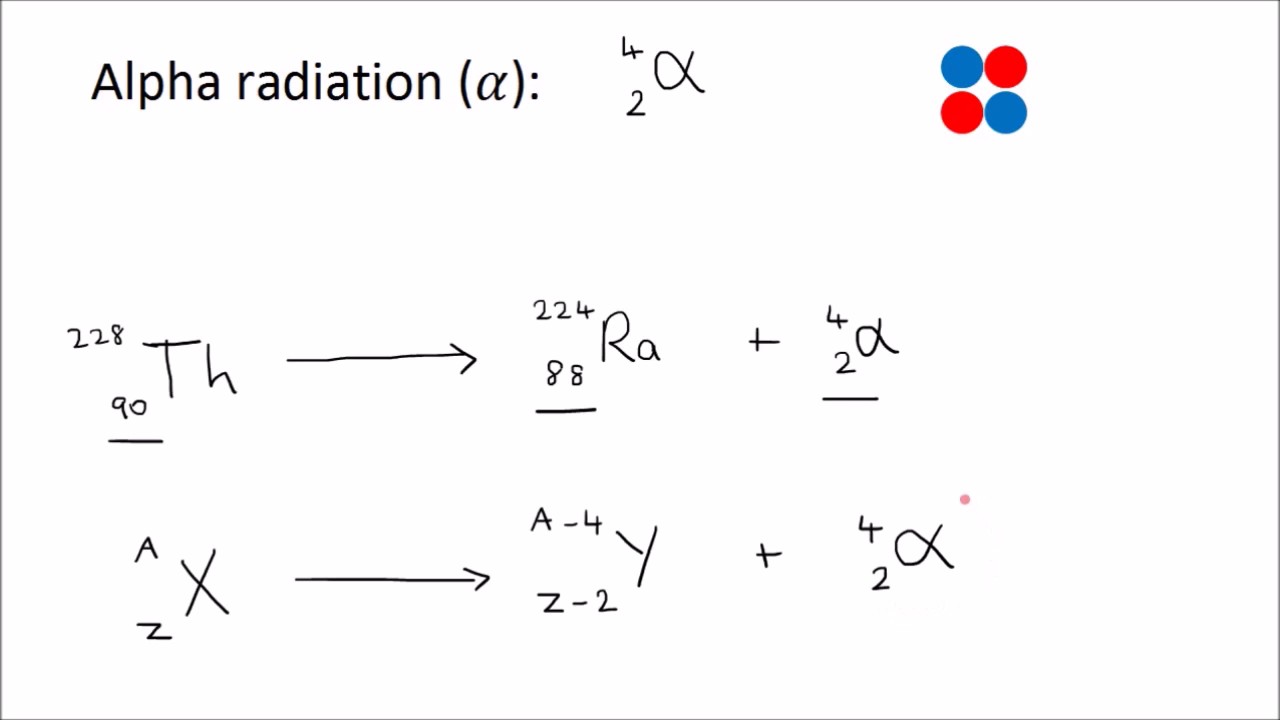
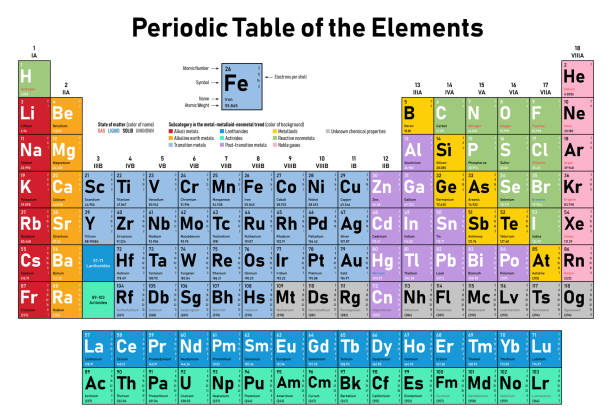
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
Atomic Cu Routing Number
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.